H4n2 Lewis Structure. 10102019 The formula for water is H 2 O. Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule. 3102011 This makes sense but when I try to convert it to a 3D structure. However the carbonate anion CO32- does have a Lewis dot structure.
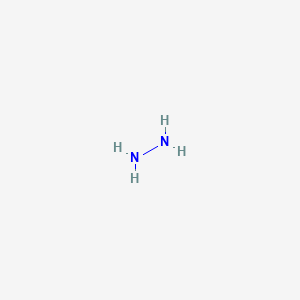
When I in charge for I we have and to has the Louis structure en end triple bond. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together there are 26 nonbonding valence electrons. Hydrazine is mainly used as a foaming agent in preparing. Lewis structures also known as Lewis dot formulas Lewis dot structures electron dot structures or Lewis electron dot structures LEDS are diagrams that show the bonding between atoms of a molecule as well as the lone pairs of electrons that may exist in the molecule. Each home pairs here Frenchy H two c n h. Its supposed to have a total of 18 electrons in the lewis dot structure it does have 18.
The same is true for the two structures you have drawn with an N N single bond.
10102019 The formula for water is H 2 O. The structure on the right side does not obey the octet duet rule because the positively-charged nitrogen only has an electron sextet. In the 3D it has 24. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together there are 26 nonbonding valence electrons. Consider the Lewis structure for sulfur tetrafluoride SF 4 which contains 34 valence electrons. Counting valence electrons yields eight total six from oxygen one each from the two hydrogens.