
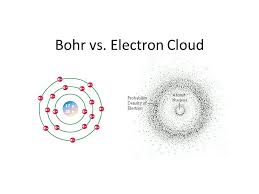
Electron Cloud Model Definition. The electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas. It is quite contrary to the traditional atomic model theory also known as Bohrs Model given by Neil Bohr. A German scientist named Werner Heisenberg proposed the idea of the electron cloud model in 1927. Click again to see term.
The model is a way to help visualize the most probable position of electrons in an atom. The electron cloud model is currently the accepted model of an atom. The electron cloud model uses the concept of orbitals referring to regions in the extra-nuclear space of an atom where electrons are likely to be found. The Electron Cloud Model explained - YouTube. Who invented the electron cloud model. 722021 The electron cloud model refers to a name given to the results of using Schrdingers equation to do a probabilistic location of an electron in a hydrogen atom.
A German scientist named Werner Heisenberg proposed the idea of the electron cloud model in 1927.
Click card to see definition. The equation was developed by Austrian theoretical physicist Erwin Schrdinger in 1926. Area around the nucleus of an atom where the atoms electrons are most likely to be found. Electron cloud model is a model of an atom in which the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas. It is quite contrary to the traditional atomic model theory also known as Bohrs Model given by Neil Bohr.